An optogenetic toolkit for light-inducible antibiotic resistance - Nature.com
Abstract
Antibiotics are a key control mechanism for synthetic biology and microbiology. Resistance genes are used to select desired cells and regulate bacterial populations, however their use to-date has been largely static. Precise spatiotemporal control of antibiotic resistance could enable a wide variety of applications that require dynamic control of susceptibility and survival. Here, we use light-inducible Cre recombinase to activate expression of drug resistance genes in Escherichia coli. We demonstrate light-activated resistance to four antibiotics: carbenicillin, kanamycin, chloramphenicol, and tetracycline. Cells exposed to blue light survive in the presence of lethal antibiotic concentrations, while those kept in the dark do not. To optimize resistance induction, we vary promoter, ribosome binding site, and enzyme variant strength using chromosome and plasmid-based constructs. We then link inducible resistance to expression of a heterologous fatty acid enzyme to increase production of octanoic acid. These optogenetic resistance tools pave the way for spatiotemporal control of cell survival.
Introduction
Antibiotic resistance genes are widely used in synthetic biology. They are included in genetic constructs to ensure plasmid propagation. Resistance genes also play an important role in cloning methods. Examples include chromosomal insertions, where expression of resistance genes can be used as a selective marker for successful integration1, or in the creation of transposon libraries, where drug resistance is used as an intermediate selection mechanism before being swapped for an alternative sequence2,3.
Although antibiotic resistance genes are a staple of synthetic biology and microbial biotechnology research, there are few methods for dynamic control of their expression. The ability to control drug resistance spatially and temporally could open new avenues for synthetic biology research. As an analogy, when Sheth et al.4 developed an inducible origin of replication—another ubiquitous feature within synthetic biology constructs—it sparked new areas of research including biological data storage5 and whole-cell riboswitch diagnostics6. Spatiotemporal control over drug resistance could enable spatial patterning in living biomaterials7, selection of single cells from microfluidic systems8,9, and improved understanding of the role dynamics play in clinical antibiotic resistance10. For example, resistance is often spread through horizontal gene transfer events11,12, which are difficult to monitor and control at the single-cell level. New systems for control offer the potential for future studies quantifying how different spatiotemporal arrangements of cells acquiring resistance can lead to population-level proliferation or collapse.
Optogenetic methods are a powerful and widely used tool for controlling gene expression13. The delivery of light to cells can be regulated in space and time, and can be integrated directly into computational workflows14,15. Optogenetic systems in bacteria have been used to control gene expression for a variety of applications13,16, including to drive metabolic flux17, regulate the gut microbiome18, control cell morphology19, and regulate co-culture dynamics20. Using light to control cell survival has been a focus of microbial engineering across species. For example, optogenetic regulation has been used to control nourseothricin resistance in Saccharomyces cerevisiae21 and bleomycin resistance in Yarrowia lipolytica22. In Escherichia coli, light has been used to control antibiotic resistance via individually designed photo-caged antibiotics23 or by leveraging the natural photosensitivity of tetracycline24. However, because these methods require careful protein engineering or exploit properties specific to a single drug, they do not easily generalize across different resistance mechanisms. An alternative approach used a light-inducible promoter to reversibly control chloramphenicol resistance20,25. Such a method could generalize to other resistance genes, however experiments were limited to control of the chloramphenicol acetyltransferase enzyme. The ideal platform for light-inducible resistance would be both generalizable for different antibiotic resistance genes and tunable across antibiotic concentrations to flexibly enable diverse studies in synthetic biology and microbiology.
To address these needs, we used the blue light-inducible Cre recombinase OptoCreVvd2 to activate antibiotic resistance genes26. Using this system, we excise a loxP-flanked transcription terminator between a gene and promoter, allowing for increased gene expression after exposure to 465 nm blue light (Fig. 1a). Recombinase technology has been used successfully for a variety of applications that require robust and inducible control of gene expression, including gene logic circuits and cell lineage tracking27,28,29. We selected this system for its relatively short activation time, flexibility in construct design (requiring only the addition of loxP sites), and minimal basal expression in uninduced cells26. In addition, the permanent OFF-ON switch caused by Cre allows for selection of resistant cells at any point after light exposure, allowing for cellular memory after the light input has been removed. Although this irreversibility does not allow for complex temporal dynamics, one-time induction provides benefits that may be advantageous in certain applications such as allowing irreversible activation before a culture becomes too dense for light penetration, or minimizing exposure in light-sensitive strains.
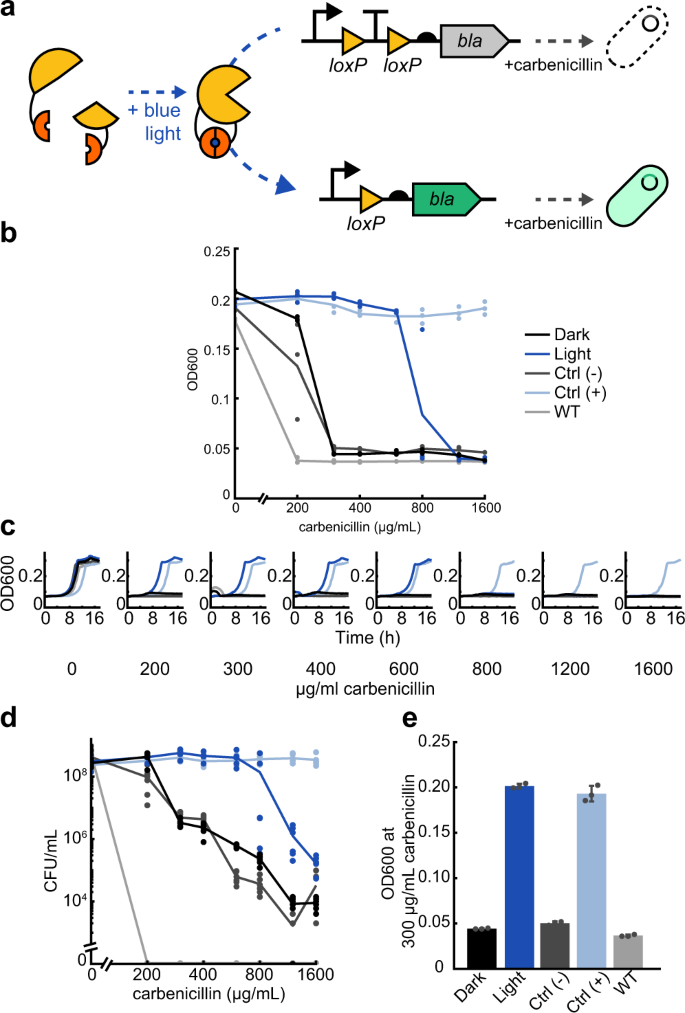
a Split Cre recombinase fragments are linked to blue light-inducible Vvd photodimer domains. When exposed to blue light, Cre becomes active and can excise a transcription terminator between two loxP domains, allowing increased expression of beta-lactamase (bla). The expression of OptoCre-bla then allows cells to survive in the presence of the antibiotic carbenicillin. b Minimum inhibitory concentration (MIC) curves of chromosomally-integrated OptoCre-bla constructs grown in carbenicillin for 18 h. Light-induced samples were exposed to blue light for 2 h immediately before exposure to carbenicillin. Growth measured by OD600 (n = 3). The strains in both the dark and light conditions contain the resistance induction construct and OptoCreVvd2. Control (−) cells contain the resistance activation construct but no Cre recombinase. Control (+) cells contain a constitutively expressed bla gene. Wild-type (WT) cells are MG1655 without modification or plasmids. c Time-course growth of OptoCre-bla resistance activation constructs across different concentrations of carbenicillin. d Colony forming unit (CFU) counts of cultures from MIC data (n = 6). After growth in carbenicillin for 18 h, samples were spotted on agar plates and colonies were counted the next day. e Optimal OptoCre-bla activation conditions. Resistance activation constructs grow in 300 µg/mL carbenicillin after exposure to blue light for 2 h, but not when kept in the dark. Growth is quantified by OD600 after 18 h. Error bars show standard deviation around the mean (n = 3 biological replicates).
Here, we used Cre to induce expression of four antibiotic resistance genes, which we selected for their ubiquity in synthetic biology applications as well as their range of mechanisms of action (Table 1). Specifically, we chose the carbenicillin/ampicillin resistance gene beta-lactamase (bla), which is both clinically relevant and widely used in synthetic biology. Beta-lactam antibiotics inhibit cell wall biosynthesis, and are enzymatically degraded by the beta-lactamase enzyme12,30,31. We also included kanamycin nucleotidyltransferase (knt), which provides enzymatic resistance against kanamycin, an antibiotic that causes mistranslation by the 30S ribosomal subunit32. Chloramphenicol acetyltransferase (cat) provides enzymatic resistance against chloramphenicol, which interferes with the 50S ribosomal subunit to cause protein synthesis to stall33. Lastly, we included the tetracycline efflux pump A (tetA) as a non-enzymatic, efflux-based resistance mechanism34. Tetracycline binds the 30S ribosomal subunit to inhibit protein synthesis35. These four antibiotics include both bactericidal (carbenicillin/ampicillin and kanamycin) and bacteriostatic (chloramphenicol and tetracycline) drugs. This selection of resistance mechanisms shows both the broad variety of mechanisms that can be controlled using this system and introduces multiple options for synthetic tools that can be incorporated into existing bacterial systems.
When controlling expression of antibiotic resistance genes, key performance metrics include the uninduced and induced expression levels. For example, when using potent enzymes like beta-lactamases, even a small amount of basal expression can allow bacterial growth in the presence of an antibiotic. Moreover, expression in the induced state should also be sufficient to provide resistance at drug concentrations comparable to typical working ranges for the antibiotic, which could further vary between use cases. To optimize these two features in our platform, we varied the gene copy number, promoter, ribosome binding site (RBS), and coding sequence to tune the minimum inhibitory concentration (MIC) of antibiotic at which cells survive after exposure to blue light, while maintaining basal expression levels that are low enough to avoid erroneously triggering survival. We further demonstrated live activation of resistance genes and characterized cellular responses using single-cell time-lapse microscopy.
Finally, we demonstrated the utility of light-inducible resistance in a biotechnology application by co-expressing resistance genes with the heterologous thioesterase CpFatB1 to increase production of octanoic acid, a medium-chain fatty acid. Medium-chain fatty acids are high-value biochemicals used in fuels, polymer production, flavorings, and fragrances, making them key metabolic engineering targets36,37. However, induction of CpFatB1 is metabolically taxing, a common issue with expression of heterologous enzymes for bioproduction applications. This challenge has prompted researchers to develop systems where pathway expression timing can be precisely tuned to balance the tradeoff between growth and production38,39. For example, light induction has been used to increase production of other chemicals in E. coli, such as mevalonate and isobutanol40. In our system with OptoCreVvd2, light induction of CpFatB1 coupled with antibiotic selection for high expression of the heterologous enzyme significantly increased fatty acid production over light induction of CpFatB1 alone.
This toolkit of light inducible resistance genes supports and extends the long use of antibiotics as cellular control mechanisms in synthetic biology, adding a spatial and temporal control mechanism to existing systems and setting the stage for future applications where light is used in combination with antibiotics to enable flexible control of cell behavior and survival.
Results
Light induction of beta-lactamase resistance
For optogenetic control of resistance genes, we used the blue light-inducible split Cre recombinase OptoCreVvd226. This system allows for excision of genetic elements placed between loxP sites when cells are exposed to blue light. Excision can be completed in ~2 h, which is comparable to or faster than many existing bacterial optogenetic systems19,25,41,42. We used OptoCreVvd2 to excise a transcription terminator placed inside loxP sites between a promoter and an antibiotic resistance gene, allowing for expression of the resistance gene only after exposure to blue light.
We first used this system to control transcription of the beta-lactamase (bla) resistance gene (which we denote 'OptoCre-bla', Fig. 1a). Beta-lactam antibiotics, including ampicillin and carbenicillin, inhibit peptidoglycan layer biosynthesis in the bacterial cell wall. Beta-lactamase enzymes can inactivate beta-lactam antibiotics by hydrolyzing the beta-lactam ring on the antibiotic43. For these studies, we used the TEM-116 beta-lactamase, which is commonly used in antibiotic resistance cassettes for plasmid selection44. We integrated this genetic construct after nupG in the E. coli MG1655 chromosome.
To measure light-induced antibiotic resistance, we exposed cultures of OptoCre-bla to blue light for 2 h, then grew them overnight in the presence of carbenicillin and compared growth to cultures kept in the dark. We observed blue light-dependent differences in cell proliferation, where the MIC necessary to prevent growth was 300 µg/mL for cultures kept in the dark and 1200 µg/mL for cultures exposed to blue light (Fig. 1b). Negative control (− Control) cells with only the reporter and no Cre recombinase had comparable survival to cells with the full construct grown in the dark, indicating low expression of bla in the uninduced state. Positive control (+ Control) cells with constitutive expression of bla from its native promoter and RBS grew in all concentrations of carbenicillin tested, including at levels above the light-inducible strain, as expected for a fully resistant strain expressing the resistance gene in its native context. We further included E. coli MG1655 as a wild-type negative control, which did not grow in any concentration of carbenicillin used here.
To confirm that blue light-induced cells grow at rates comparable to the positive control strain, we collected time-series data demonstrating normal growth rates under a broad range of carbenicillin concentrations for cells grown in blue light, while cells without light induction failed to grow (Fig. 1c). We further validated the optical density-based MIC data by using colony forming unit (CFU) counts after antibiotic exposure (Fig. 1d). Although MIC data is an accurate assessment of cell growth in the presence of antibiotic, beta-lactam antibiotics also cause cell filamentation, which can increase optical density (OD) readings even when cells are not dividing, making bla resistance specifically important to confirm by CFU measurement45. However, our results using CFU counts confirm that the optical density measurements also translate to a clear difference in cell survival46. Overall, we found that OptoCreVvd2 can be used to induce beta-lactamase resistance and we identified concentrations of carbenicillin with robust differences between dark and blue-light activated expression of the bla resistance gene construct (Fig. 1e and Table 2).
Optimization of kanamycin resistance
We next sought to generalize this system to other antibiotic resistance genes. While the excision of a terminator can easily couple light to the expression of an antibiotic resistance gene, this is not the same as light-inducible survival. Control of survival requires no or low expression of the antibiotic resistance gene in the dark, such that cells remain susceptible to antibiotics. The design also requires that induction of the resistance gene is sufficient to confer resistance. The thresholds for these two features can vary dramatically with different antibiotic resistance mechanisms, which have different rates of antibiotic degradation and export. Thus, while previous work in our lab has shown that OptoCreVvd2 can control expression of a fluorescent protein with low basal expression and a 10-fold change with light26, naively replacing the fluorescence gene with an antibiotic resistance gene may not produce the desired behavior. Therefore, we defined a general process for adapting the OptoCreVvd2 system to different antibiotic resistance genes (bla, knt, cat, and tetA) and were able to show survival at customized ranges of antibiotic concentration.
To generalize our system to other antibiotics, we began by exchanging bla for kanamycin nucleotidyltransferase (knt) in our induction construct to make OptoCre-knt (Fig. 2a). The antibiotic kanamycin causes mistranslation by binding to the 30S subunit of the bacterial ribosome. The knt enzyme catalyzes transfer of a nucleotide to kanamycin, inactivating the antibiotic32. Although our initial design of OptoCre-knt did show resistance activation using light, the kanamycin concentration required to see this difference was very high – over 1000 µg/mL (Fig. 2b), compared to 25–50 μg/mL commonly used for plasmid propagation24,44. Although matching common working concentrations of antibiotics is not essential, using concentrations in the vicinity of these ranges provides benefits including the ability to study community effects at physiological concentrations and limiting overall antibiotic needs.
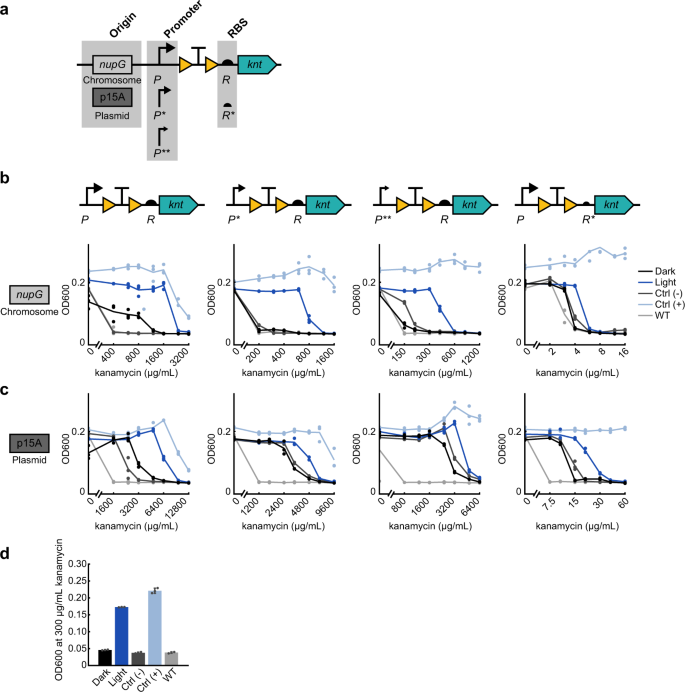
a Expression levels of the kanamycin resistance gene knt can be tuned by changing the strength of the promoter and RBS, as well as the origin of replication. Promoter strength ranges from P (medium) to P* (medium-low) to P** (low). RBS strength ranges from R (strong) to R* (weak). b MIC curves of OptoCre-knt activation cassettes on the chromosome, with promoter P, P*, or P** and RBS R or R* (n = 3). c MIC curves of OptoCre-knt activation cassettes on a plasmid with the p15A origin of replication (n = 3). d Optimal OptoCre-knt activation conditions, using P* and R on the chromosome at 300 µg/mL kanamycin. Growth is quantified by OD600 after 18 h. Error bars show standard deviation around the mean (n = 3 biological replicates).
Thus, we set out to tune gene expression through optimization of the genetic architecture surrounding the gene. To lower basal expression, we weakened the promoter or RBS driving knt expression. By changing the promoter and RBS of OptoCre-knt, we were able to shift the expression of the resistance gene to allow survival at antibiotic concentrations much closer to the MIC of wild-type MG1655 (Fig. 2b). We tested a range of promoter and RBS combinations to show how these alterations impact survival at varying antibiotic concentrations. We used constitutive promoters of varying strength all based on the T7A1 viral promoter, ranging from medium, P, to medium-low, P*, to low, P**, transcriptional strength. We also used the RBS of gene 10 in the T7 phage47, which we denote R, and a RBS that we computationally designed to be weaker48, which we denote R* (Fig. 2a). Changing P to P* decreased the MIC for the dark state to 200 µg/mL kanamycin, while P** further reduced it to 150 µg/mL (Fig. 2b). The MIC for the light state was also reduced, as expected, but still maintained a wide range of kanamycin concentrations resulting in survival. With P*, kanamycin levels between 200 and 800 µg/mL resulted in light-induced survival, while for P** the range was from 150 to 500 µg/mL. Using R* in combination with P caused a dramatic decrease in both the dark state MIC and the effective concentrations for light-induced survival, resulting in a narrow range between 4 and 6 µg/mL kanamycin.
Although the chromosomally integrated constructs used so far have the advantages of low background expression and do not require a selection marker, plasmids offer their own advantages for light-inducible resistance systems. Many resistance genes are naturally found on plasmids, and a plasmid origin allows for convenient transfer of systems between different strains. We further characterized our constructs on plasmids containing the p15A origin of replication, which has approximately ten copies per cell (Fig. 2c)49. Changing from chromosomal integration to a p15A plasmid increased the range of antibiotic concentrations at which cells containing OptoCre-knt selectively survive by over 5-fold. Despite this increase, we found that strategies such as lowering promoter or RBS strength can have a counterbalancing effect. We also characterized a p15A plasmid-based OptoCre-bla, however its basal resistance was too high to be considered functional (Supplementary Fig. 1a). Overall, the plasmid-based system with OptoCre-knt removes the need for the chromosomal insertion process, increasing the ease at which these constructs can be used in different strains or contexts.
The flexibility afforded by these different designs led us to develop multiple constructs, and the optimal construct is likely to be application-specific. For example, the lower concentrations shown here are near the wild-type MIC, which is optimal for studies looking to characterize resistance acquisition using phenotypically-relevant antibiotic concentrations. In contrast, the higher concentrations allow more stringent cell selection for studies where only the activated cells should survive. Through this optimization process, we found constructs that allow a greater kanamycin MIC fold change between dark and light-exposed cultures compared to our original construct, notably P* and R driving OptoCre-knt expression on the chromosome is an ideal example of our optimized design (Fig. 2d and Table 2).
Protein-level optimization of chloramphenicol resistance
Light-induced survival requires a tight OFF-state where cells are susceptible to antibiotic. As we have demonstrated, this can be achieved with low basal expression of the resistance gene. However, the uninduced state can also be minimized if the resistance enzyme itself is weaker. Thus, when activating the chloramphenicol acetyltransferase (cat) enzyme, we took advantage of a known mutation to decrease the strength of the enzyme itself. Chloramphenicol prevents protein synthesis by binding to the 50S ribosomal subunit, where it inhibits peptide bond formation. The cat enzyme prevents chloramphenicol from binding to the ribosome by attaching an acetyl group from acetyl-CoA to the antibiotic50. By using the weaker catT172A variant51,52, we lowered the concentration of antibiotic at which cells survive (Fig. 3a). In the OptoCre-cat design, we used the promoter P and RBS R, and compared light-induced survival by cat and catT172A. Here we observed a sharper decrease in dark OFF-state resistance with catT172A compared to cat, lowering basal resistance to that of the wild-type strain on a chromosomally integrated construct (Fig. 3b). We also observed a decrease in MIC values for cat and catT172A on a p15A plasmid origin (Fig. 3c). This enzyme mutant approach to optimization may be particularly helpful when working with enzymes that show resistance to high concentrations of antibiotic even with minimal basal gene expression, or if using this system on a plasmid with a high copy number where it is hard to limit basal expression. This approach creates another point at which resistance levels can be fine-tuned, and the light-induced growth difference shown by catT172A on a p15A plasmid origin is a particularly versatile optimized design (Fig. 3d and Table 2).
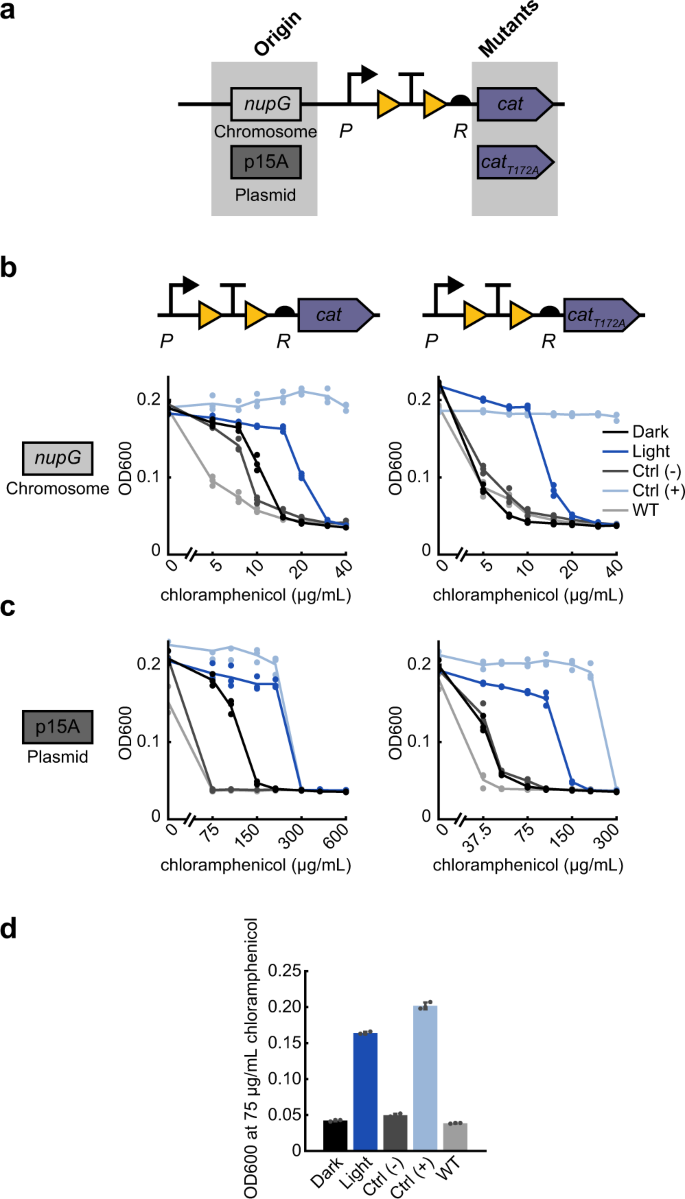
a Resistance given by the chloramphenicol resistance gene cat can be lowered by using the catT172A variant on a chromosomal or plasmid origin. b MIC curves of OptoCre-cat activation cassette with the native enzyme cat or weakened enzyme catT172A on the chromosome (n = 3). c MIC curves of the cat and catT172A activation cassettes on a plasmid with the p15A origin (n = 3). d Optimal activation conditions, using catT172A with promoter P and RBS R on a plasmid origin at 75 µg/mL chloramphenicol. Growth is quantified by OD600 after 18 h. Error bars show standard deviation around the mean (n = 3 biological replicates).
We wondered whether it would be possible to tune resistance levels by adjusting light exposure properties. To test this, we further characterized the OptoCre-cat design by modifying light exposure duration and intensity (Supplementary Fig. 2). We found that resistance levels were tunable, and depended on a combination of both duration of light exposure and the intensity of the blue light.
A common issue with many of our designs is that basal expression results in resistance levels that, although low, still exceed those observed in the wild-type strain. In principle, this leakiness could be the result of several different issues including spontaneous recombination events, mutations in the terminator, or readthrough of the terminator. We sequenced the loxP and terminator region of the (-) Control strain without Cre from both 0 µg/mL chloramphenicol and 75 µg/mL chloramphenicol conditions, where the latter represents the highest antibiotic concentration condition that showed any growth. Sequencing results from both conditions matched the original sequence of the plasmid, confirming that leaky expression is not the result of spontaneous recombination or terminator mutations. These results are in line with characterizations of the original OptoCreVvd2 design, which showed consistent low basal expression of a fluorophore with no evidence of spontaneous recombination26. To mitigate the potential for terminator readthrough we then attempted to lower basal expression by swapping the strong BBa_B0015 terminator from our original design to the synthetic terminator L3S2P21, which was the strongest terminator identified in an extensive set characterized in Chen et al.53. However, this did not show further improvement over the terminator used in our initial design (Supplementary Fig. 3), so we did not pursue this avenue further. For applications where minimal basal expression is critical, alternative designs could test other terminators53,54 or different circuit design approaches55,56,57.
Efflux pump enabled tetracycline resistance
Light induction can also be applied to non-enzymatic antibiotic resistance mechanisms, such as the tetA efflux pump. The antibiotic tetracycline reversibly binds to the 30S ribosomal subunit, inhibiting protein synthesis. The tetA efflux pump localizes to the inner membrane and exports magnesium-tetracycline chelate complexes by importing a proton35. In sharp contrast to bla, knt, and cat where the native resistance levels were high and our engineering efforts aimed at reducing potency, we found that our initial design for inducible tetA did not show resistance over wild-type MG1655 when expressed with promoter P and RBS R on a p15A origin plasmid (Supplementary Fig. 1b), conditions which produced the highest levels of resistance in the constructs we tested previously. To compensate for this, we opted to use the strong native promoter Ptet with its corresponding RBS Rtet to allow full expression of the tetA gene58 to create OptoCre-tetA (Fig. 4a). Using this native architecture, OptoCre-tetA showed some activation when chromosomally integrated (Fig. 4b), and exhibited strong activation on the p15A plasmid origin (Fig. 4c). Notably, the dark-state basal resistance over wild-type MG1655 was minimal, allowing for activation of OptoCre-tetA at low tetracycline concentrations. Thus, we found that the p15A plasmid-based version is an ideal construct for tetracycline resistance (Fig. 4d and Table 2).
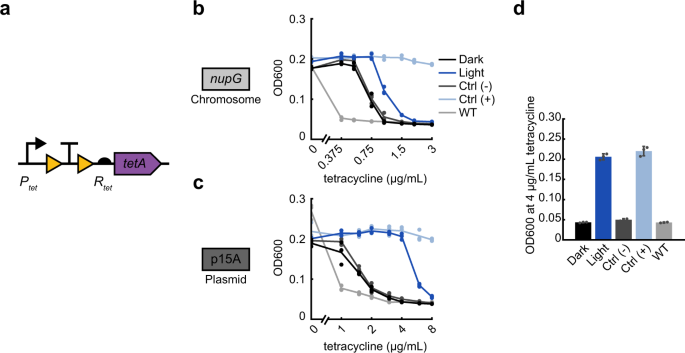
a Tetracycline resistance gene tetA is expressed using the native promoter Ptet and native RBS Rtet, to allow maximal expression of the gene. b MIC curves of OptoCre-tetA on the chromosome and c p15A plasmid origin (n = 3). d Optimal OptoCre-tetA activation conditions, using the p15A plasmid origin at 4 µg/mL tetracycline. Growth is quantified by OD600 after 18 h. Error bars show standard deviation around the mean (n = 3 biological replicates).
Single-cell microscopy showing resistance activation
The spatial and temporal precision enabled by optogenetics allows these constructs to be used for a variety of applications, including single-cell studies of bacterial antibiotic resistance. How resistance acquisition leads to bacterial survival at the single-cell level is of particular interest in the context of horizontal gene transfer. Existing single-cell horizontal gene transfer studies have previously characterized gene transfer rates and shown important connections to quorum sensing59,60. However, natural instances of horizontal gene transfer are infrequent and difficult to control in space and time, especially relative to antibiotic exposure. Previous studies have also shown that stochastic acquisition of resistance in single cells is not always enough to cause the proliferation of phenotypically resistant cells61. To study when horizontal gene transfer events lead to the spread of resistance in populations, it would be interesting to model a single cell's acquisition of resistance with optogenetic control of antibiotic susceptibility. This could be used to characterize when and how resistance acquisition in single cells leads to antibiotic evasion, and how specific antibiotic dosing schedules and concentrations impact evasion frequency.
Here we show a proof-of-concept for the first steps in this class of studies by inducing cell growth using blue light for cells on agarose pads. Using time-lapse microscopy, we placed cells containing light-activatable antibiotic resistance on agarose pads containing antibiotics and compared the growth of cells that were kept in the dark to those exposed to blue light. For these studies we elected to focus on a subset of resistance genes, selecting kanamycin and chloramphenicol as examples of bactericidal and bacteriostatic antibiotics, respectively. We characterized resistance activation for chromosomally integrated OptoCre-knt resistance to kanamycin (Fig. 5a and Supplementary Movie 1), and plasmid-based OptoCre-cat resistance to chloramphenicol (Fig. 5b and Supplementary Movie 2). We found that cells with light-induced resistance showed a short lag before growth compared to their constitutively resistant positive controls, which likely corresponds to the time needed to excise the transcription terminator and allow expression of the resistance gene. In contrast, when cells were kept in the dark, growth was inhibited and we observed examples of loss of membrane integrity (Supplementary Movie 1–2). To quantify recovery of resistance-induced cells, we calculated the percentage of cells in the initial frame that recovered over the course of the movie (Supplementary Fig. 4). We defined the time of recovery as the point at which a cell first divides, and found 70% recovery for OptoCre-knt and 42% recovery for OptoCre-cat with light exposure (compared to 13% and 4% for cells in the dark, though notably cells in the dark rarely experienced more than one division event, Supplementary Movie 1–2). Although these data show clear differences between light and dark exposure, the incomplete rates of recovery under light exposure may be due to simultaneous exposure to antibiotic and light, likely rendering some cells nonviable before they can be induced. In the future, microfluidic experiments could help to assess recovery rates as a function of relative light induction and antibiotic addition timing.
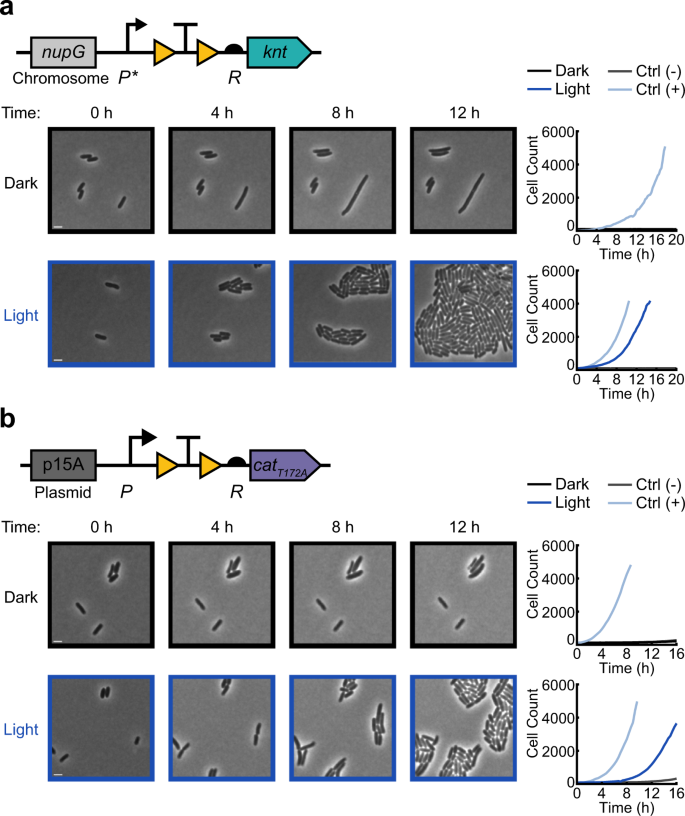
Activation of a the chromosomal OptoCre-knt resistance construct with promoter P* and RBS R on agarose pads containing 400 µg/mL kanamycin, and b the p15A plasmid-based OptoCre-cat resistance construct using catT172A with promoter P and RBS R on agarose pads containing 60 µg/mL chloramphenicol. Microscopy images show representative samples of the resistance activation strains in the dark or with blue light (scale bar = 2 µm). Cell counts over time are cumulative across multiple imaging positions for each condition, with each plot containing the OptoCre resistance strain along with negative and positive controls (n = 3 biological replicates).
Looking towards future applications that require the control of subpopulations of cells, we also used a digital micromirror device (DMD)62 to activate resistance in a subset of cells. The DMD allows for programmed illumination of specific areas within a field of view. We illuminated half of the field of view and saw preferential activation of cells in the blue light illuminated region (Supplementary Fig. 5). We did observe some growth in a subset of the cells in the dark half of the field of view, albeit at reduced levels relative to the illuminated half. This may be due to diffusion of light from the DMD, as we do not see this activation until after DMD illumination starts. Compared to the longer, lower intensity exposures we used in liquid culture experiments, the DMD is designed to deliver intense periods of light. These differences suggest that DMD activation protocols and setups could be optimized for improved activation timing in the future.
Improving octanoic acid production using antibiotic resistance selection
To demonstrate the utility of the system for biotechnology applications, we next focused on increasing yield of octanoic acid through co-expression of cat or tetA with the thioesterase CpFatB1 (Fig. 6a-b). CpFatB1 is derived from the plant species Cuphea palustris and has been optimized for expression in E. coli63. CpFatB1 expression is taxing on cells, therefore directly coupling its induced expression with antibiotic resistance can allow for selective growth of cells actively producing both enzymes, thereby preventing the growth of non-expressing cells (Fig. 6c). We used OptoCre-cat with the catT172A mutant and OptoCre-tetA for selection due to their wide induction ranges and minimal basal resistances on the p15A plasmid backbone. For fatty acid production, we used the highly active variant CpFatB1.2-M4-287 which has been previously shown to boost octanoic acid production63. To optimize expression of the CpFatB1 gene without disrupting the resistance activation architecture, we placed it immediately downstream of the resistance gene under expression of a strong computationally-designed RBS denoted R′. Consistent with the protocol for inducing antibiotic resistance alone, we performed induction by exposing cells to blue light for 2 h early in the growth phase. From a bioproduction perspective, an advantage of the OptoCreVvd system is its irreversibility, which enables permanent activation of CpFatB1 without the need for continuous illumination. This circumvents issues with dynamic light induction systems, where light penetration can become a concern for dense cell cultures in metabolic engineering contexts40,64. We found that light induction alone led to a significant increase in octanoic acid production for both the cat and tetA systems. We next asked whether introducing antibiotic selection could further improve yields. We found that with OptoCre-cat, adding 150 µg/mL chloramphenicol significantly improved production, increasing it by 29% over the light induction-only condition, with higher chloramphenicol concentrations resulting in similar performance (Fig. 6d). Light induction alone or with antibiotic also showed a substantial improvement in yield over a constitutively expressed version of CpFatB1 with identical genetic architecture and constitutive Cre recombinase expression (Fig. 6d). With OptoCre-tetA, octanoic acid production increased 150% over light induction alone when it was supplemented with 1.5 µg/mL tetracycline (Fig. 6e). Higher tetracycline concentrations improved yield further, reaching a 300% increase over light alone when 6 µg/mL tetracycline was added. These concentrations are on the upper range of the resistance levels we see in our MIC experiments for the respective strains, possibly due to the higher OD (OD600 ≈ 0.6) at the time antibiotics are added in our bioproduction protocol. The induction system with OptoCre-tetA did not boost production over constitutive expression of CpFatB1, though the addition of antibiotic did greatly improve yield over light alone (Fig. 6e). Importantly, the constitutively expressed versions of both the cat and tetA constructs showed poor growth profiles compared to the light inducible versions (Supplementary Fig. 6). This growth deficit in the constitutive constructs is problematic, as it may lead to escape mutants and reduce the stability of production strains. Thus, coupling octanoic acid production with resistance selection leads to higher yields of octanoic acid than light induction alone, without the taxing growth deficit associated with continuous production.
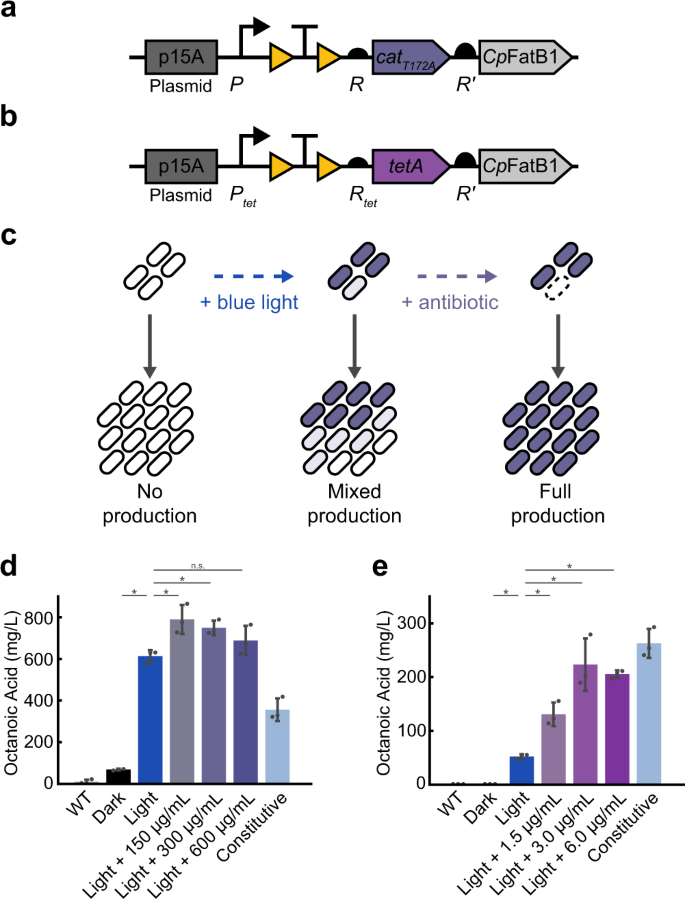
a Activation of OptoCre-cat using catT172A or b OptoCre-tetA is coupled with CpFatB1 by introducing the gene downstream of the drug resistance marker under the strong RBS R'. c Blue light induces expression of the resistance gene and CpFatB1, but does not guarantee all individuals within a community are expressing the genes. Non-producers have a growth advantage due to the burden of CpFatB1. Addition of chloramphenicol prevents growth of any individuals not producing the resistance gene and consequently CpFatB1. d Octanoic acid production coupled with OptoCre-cat measured by GC-MS. e Octanoic acid production coupled with OptoCre-tetA measured by GC-MS. Significance was determined using a two-tailed Welch's t-test: *P < 0.05; n.s. not significant. Error bars show standard deviation around the mean (n = 3 biological replicates).