A score to predict Pseudomonas aeruginosa infection in older patients with community-acquired pneumonia - BMC ... - BMC Infectious Diseases
Even while P. aeruginosa only causes severe CAP in only 1.8% to 8.3% of patients, it contributes to a high mortality rate of 50% to 100% [4]. Therefore, empiric therapy for patients with suspected P. aeruginosa infection is necessary to reduce mortality.
According to country-specific treatment guidelines, the risk factors for P. aeruginosa pulmonary infection vary. These include severe underlying lung disease, recurrent bronchiectasis, use of antibiotics within the previous 3 months, recent hospitalization, airway P. aeruginosa colonization, history of antibiotic therapy for 2 or more days within the previous 90 days, current tube feeding, and alcohol consumption [12,13,14,15,16,17,18].
A history of respiratory isolation of P. aeruginosa during the previous year and hospitalization with taking parenteral antibiotics within the preceding 90 days are both identified as risk factors for P. aeruginosa infection in the most recent 2019 treatment guidelines from the IDSA and ATS [1]. However, they stress that the most crucial ones are locally confirmed risk factors for P. aeruginosa infections because prior research from various population and geographic studies has produced inconsistent results about the associations between risk factors and P. aeruginosa infections.
The following risk factors were of particular interest: infection with or colonization by P. aeruginosa within the past year (OR 16.10; 95%CI 9.48–27.35) [6], enteral tube feeding (OR 13.87; 95%CI 3.39–56.65) [8], tracheostomy (OR 6.50; 95%CI 2.61–16.19) [6], hospitalization for more than 2 days within the past 30 days but not within the past 7 days (OR 3.8; 95%CI 1.8–8.3) [19], male (OR 3.71; 95%CI 1.65–8.35) [4], and immunodeficiency (OR 1.39; 95%CI 1.22–1.58) [20]. Despite definitional differences, the majority of studies have found that bronchial or pulmonary diseases, including asthma, uncomplicated chronic bronchitis, COPD, bronchiectasis, and interstitial lung disease, are risk factors for P. aeruginosa infection (OR 1.25–5.8; p < 0.05) [4, 6, 8, 19]. In addition, severe COPD or pneumonia necessitating mechanical ventilation or vasopressors, and having a pneumonia severity index (PSI) risk class of IV–V, are considered high risk (OR 1.85–3.95) [4, 6, 20]. Interestingly, recent exposure to inhaled corticosteroids within the past 90 days (OR 1.40; 95%CI 1.23–1.61), receiving Gram-positive coverage therapy within the past 90 days (OR 1.37; 95%CI 1.01–1.87), prior hospitalization within the past 90 days (OR 1.36; 95%CI 1.21–1.54), and use of beta-lactams within the past 90 days (OR 1.31; 95%CI 1.14–1.51) are weakly associated with P. aeruginosa infection [19]. One study also discovered that having diabetes (OR 0.82; 95%CI 0.70–0.95) and being older than 84 years old (OR 0.64; 95%CI 0.52–0.78) decreased the likelihood of contracting this pathogen [20].
One benefit of this study was the tool's ability to precisely examine risk factors for P. aeruginosa infection while various predictive scores were developed to evaluate the risk of infection from not only P. aeruginosa but also methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, Stenotrophomonas maltophilia, enterococci, and Acinetobacter baumannii, in patients with community-onset, healthcare-associated pneumonia [21,22,23,24,25,26,27,28,29,30]. Although not all of the studies listed in Table 5 provided evidence that our identified risk factors, such as NG tube feeding, bronchiectasis, or immunocompromised, were risk factors for P. aeruginosa infection, we believed that our instrument developed based on locally validated risk factors that have been shown to influence P. aeruginosa infection in patients over 60 years of age diagnosed with CAP. Another advantage was that only our study found a link between pseudomonal pneumonia and lung bleb, atelectasis, and pulmonary fibrosis.
This study identified NG tube feeding prior to admission as the strongest predictor of P. aeruginosa infection. The organism can be found in the environment, particularly in water. After entering the body via the respiratory tract, biofilms are easily formed on the inner surface of tubes [31]. In one study, P. aeruginosa was cultured from the tongue dorsal swabs of 34% of elderly patients who wore NG tubes for at least 2 weeks, whereas no such bacteria were found in the group without NG tubes (50 cases; p < 0.001). Scanning electron micrography analysis of samples from the oropharyngeal section of the NG tube revealed that the biofilm was produced by the same strain of P. aeruginosa found in the oropharynx of P. aeruginosa-infected patients [32]. NG tube feeding being a significant risk factor for P. aeruginosa is thus not surprising.
Due to primary antibody deficiencies, bronchiectasis is a risk factor for the development of CAP [33], and P. aeruginosa is the leading cause of this disease. When it binds to the airway epithelium with its flagella and pili [34], P. aeruginosa secretes various virulence factors that promote cell adhesion and tissue invasion, inhibit mucociliary function, and dysregulate host immunity, leading to airway inflammation and tissue damage. With bronchiectasis, infection and inflammation occur simultaneously in the trachea. This creates favourable conditions for the colonization of pathogens, particularly P. aeruginosa [35], through biofilm formation [34], with the severity of inflammation and the amount of colonization correlated with the severity and frequency of bronchiectasis exacerbations [35].
Immunocompromised patients, particularly those with neutrophil counts less than 500/mm3, haematologic malignancies, transplant recipients, and HIV infection, are at increased risk for P. aeruginosa infection of the pulmonary and circulatory system [34, 36, 37] due to loss of mucosal barriers, mucositis from chemotherapy, and selective pressure from broad-spectrum antimicrobial therapy [36].
The majority of the research participants with P. aeruginosa infection experienced atelectasis (73.08%). This anomaly encourages the production of biofilms, making it a risk factor for P. aeruginosa infection.
COPD prevalence did not differ between the P. aeruginosa and non-P. aeruginosa groups, ruling out a link between COPD and P. aeruginosa infection in this study. P. aeruginosa is typically detected in the sputum of 4% to 15% of COPD patients without a pulmonary infection. In the lungs of COPD patients, there are two types of colonization: short-term colonization followed by eradication and long-term persistence [38]. COPD patients with pulmonary P. aeruginosa infection are associated with a higher incidence of acute COPD exacerbations (AECOPD). It also causes chronic infections in the aforementioned patients. Studies indicated that P. aeruginosa infection can persist in the lungs of COPD patients for up to a year. Compared to bloodstream isolates from non-AECOPD patients, respiratory samples from AECOPD patients tend to have lower cytotoxicity and motility but produce more biofilm in chronic infections [39].
This study was unable to establish a correlation between a previous infection or colonization with P. aeruginosa and the risk of developing a P. aeruginosa infection. As this factor was present in only 9 of 185 patients (4.86%) and all cases were infected with P. aeruginosa, it was not possible to calculate the OR for comparing the presence of these risk factors for infection with P. aeruginosa or other pathogens.
This study has limitations due to the relatively small number of participants, primarily because a high percentage (44.90%) of patients diagnosed with pneumonia in our setting had no bacterial growth in sputum culture. This result was in line with those of a retrospective cohort research carried out at a university hospital in Thailand, which revealed that no bacteria were discovered in sputum cultures 55.15 percent of the time [40]. Furthermore, it was observed that some patients who were initially included in the study were later excluded due to concomitant infections in other organs or insufficient data for identifying risk factors, accounting for 22.00% and 4.00%, respectively.
We allocated scores of 4, 2, 2, and 1 for NG tube feeding prior to admission, bronchiectasis, immunosuppressed state, and other chronic respiratory disease, respectively, nevertheless, the cut-off score for the risk of P. aeruginosa infection was only 2 points. With the exception of having atelectasis, pulmonary fibrosis, and lung bleb, patients are at risk for contracting P. aeruginosa infection even if they only have one risk factor. Therefore, we changed the risk score to the 60-B-r-I-NG checklist (as shown in Fig. 5). CAP cases with NG tube feeding, bronchiectasis, and immunocompromised status should receive empirically antipseudomonal agent based on local susceptibility. If they merely have atelectasis, pulmonary fibrosis, or lung bleb, they do not require antipseudomonal agent.
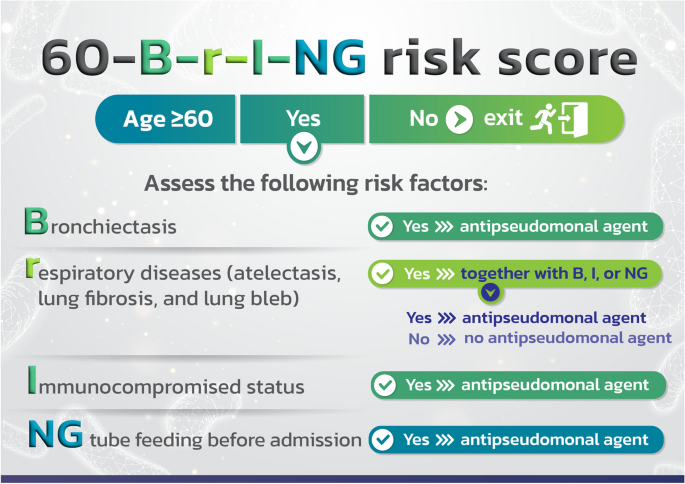
The risk score to the 60-B-r-I-NG checklist
Comments
Post a Comment